- Merkel set for fourth term as chancellor as CDU leads parliamentary vote, exit polls say
- Turkey says it will send back ISIS prisoners even if citizenships revoked
- General Marchenko: ‘Mykolaiv was to be next city to fall, but Russia terribly failed’
- Ukrainian Armed Forces repel enemy attacks in four regions
- Somalia president hails lifting of arms embargo as government vows to wipe out al-Shabab militants
- Captured Somali pirates arrive in India to face trial over ship hijacking
- Martyred while serving nation: President Raisi dies in helicopter crash
- Stay Connected
Turkey approves emergency use of Sinovac’s COVID-19 vaccine
14 January, 2021 |
Filed under: All News,more news,Opinion,RECENT POSTS,Somali news |
Posted by: Abdillahi
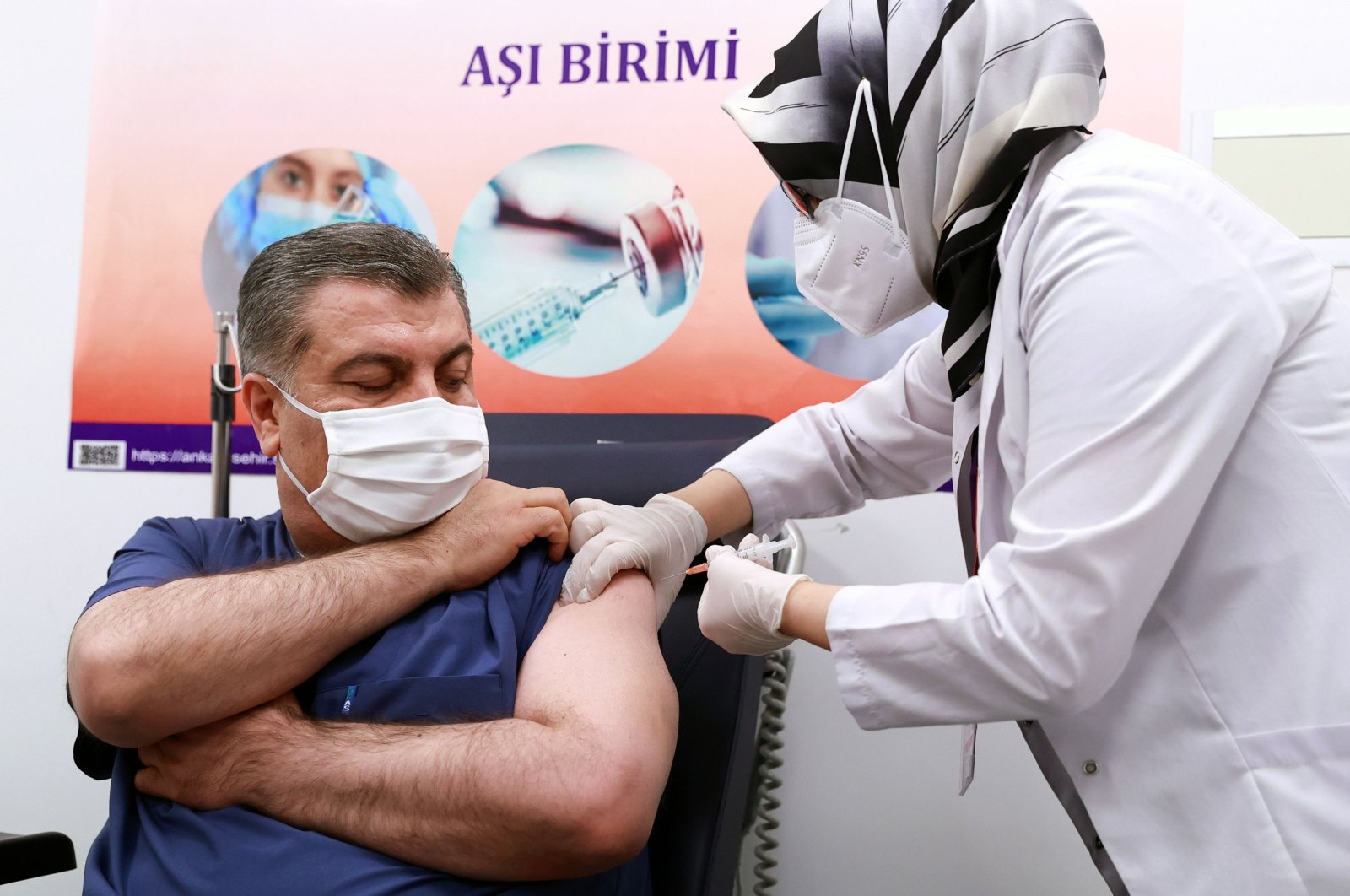
Turkey’s Health Minister Fahrettin Koca receives a dose of Sinovac’s COVID-19 vaccine
at Ankara City Hospital, in Ankara, Turkey, Jan. 13, 2021. (AFP Photo)
at Ankara City Hospital, in Ankara, Turkey, Jan. 13, 2021. (AFP Photo)
Turkey’s Medicines and Medical Devices Agency (TITCK) on Wednesday approved the emergency use of Chinese biopharmaceutical company Sinovac’s coronavirus vaccine.
The approval came after a 14-day testing period, which began when the first shipment of the vaccine, containing 3 million doses, arrived in the country on Dec. 30.
“Following scientific inspections and evaluations, the vaccine has been authorized for emergency use,” TITCK said in a statement.
The samples of the vaccine “CoronaVac,” developed by China’s Sinovac Biotech, were tested in accordance with the routine quality control processes applied all over the world, the Ministry of Health previously said in a statement.
Analyses were conducted to check the expected quality, effectiveness and reliability of the vaccine when it is stored and applied under conditions defined by the company throughout its shelf life.
Speaking in a press conference shortly after the announcement, Health Minister Fahrettin Koca said Turkey’s vaccination campaign would start Thursday.
“Starting tomorrow (Thursday), all of our health personnel will start receiving vaccines. Our citizens will be able to track the progress of the vaccination campaign on our website. We aim to have a transparent process,” Koca said.
The minister explained that each vaccine would be assigned to a person for inoculation and the QR codes on the boxes would prevent the shots from being injected into someone else.
“We want to assure our people that the distribution of the vaccines will be just and in accordance with guidelines created by the Coronavirus Science Board. The people who get their turn will be notified and they will schedule an appointment to receive their shots,” he said.
After the press conference, Koca and the members of the science board visited a nearby hospital in the capital Ankara to receive their shots in a live broadcast.
Speaking after receiving his shot, Koca said the start of the vaccination campaign marked the beginning of “brighter days.”
“Because the most effective way to protect against this disease is with vaccines. We need to all get vaccinated to return to our normal lives,” he said.
The vaccine will be administered by the Health Ministry’s COVID-19 vaccine application units, which will be established in family health centers, as well as in private and university hospitals, according to the Health Services Directorate.
Source:dailysabah.com
All News
- Lebanon’s housing sector crumbles, rental demand surges amid Israel-Hezbollah war
- Russia Fired Experimental Hypersonic Missile at Ukraine in Response to Western Long-Range Missiles – Putin
- ICC slaps Netanyahu, Gallant with arrest warrants for war crimes
- Donald Trump expected to consider recognizing Somaliland independence, former UK defense minister says
- US embassy in Kyiv shutters after ‘significant’ air attack threat By Tamsin Paternoster
- Opposition leader wins Somaliland presidential contest
- Israeli strikes rattle Beirut, as peace talks begin to take shape
- Zelenskyy says ‘missiles will speak for themselves’ as Biden OKs long-range use
- AP photographer captures moment bomb hits apartment building in Lebanon
- Two Somali Pirates Get 30 Years in Prison for Kidnapping a Journalist
- Home
- Merkel set for fourth term as chancellor as CDU leads parliamentary vote, exit polls say
- Turkey says it will send back ISIS prisoners even if citizenships revoked
- General Marchenko: ‘Mykolaiv was to be next city to fall, but Russia terribly failed’
- Ukrainian Armed Forces repel enemy attacks in four regions
- Somalia president hails lifting of arms embargo as government vows to wipe out al-Shabab militants
- Captured Somali pirates arrive in India to face trial over ship hijacking
- Martyred while serving nation: President Raisi dies in helicopter crash
- RSS
Contact@kasmaal.com